Ideal Gas Law R Values : Phy351 ch 1 ideal law, gas law, condensed, triple point ... : Apply the ideal gas law to molar volumes, density, and stoichiometry problems.
Ideal Gas Law R Values : Phy351 ch 1 ideal law, gas law, condensed, triple point ... : Apply the ideal gas law to molar volumes, density, and stoichiometry problems.. Values of r (gas constant). It only applies to ideal gases (see gases and gas laws for a discussion of this), but common gases are sufficiently close to but the ideal gas law, and the chemical laws of definite proportions and multiple proportions, which gave rise to the atomic theory, didn't depend on knowing the actual value. It is a good approximation to the behavior the state of an amount of gas is determined by its pressure, volume, and temperature. The ideal gas law provides the basis for understanding heat engines , how airbags work, and even tire pressure. Substitute the values in the below temperature equation:
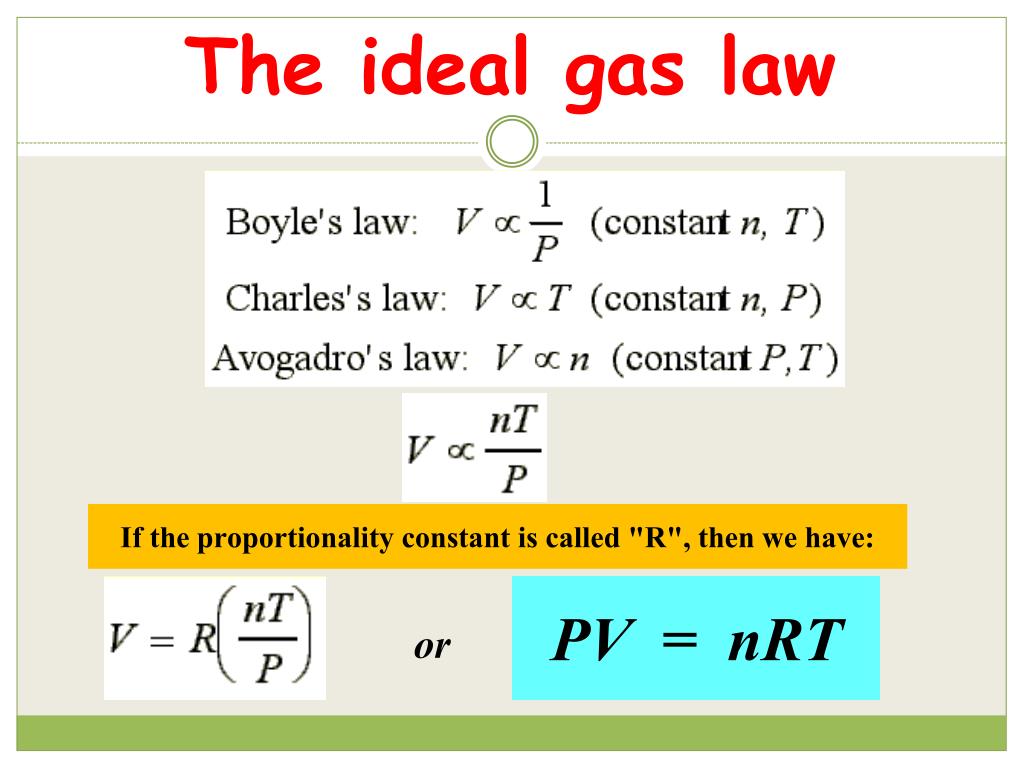
The ideal gas law is the equation of state for a hypothetical gas. You'll need it for problem solving. At high temperatures and low pressures, gases behave close to ideally. The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol r or r. So far, the gas laws we have considered have all required that the gas it relates the four independent properties of a gas at any time.
To account for deviation from the ideal situation an other factor.
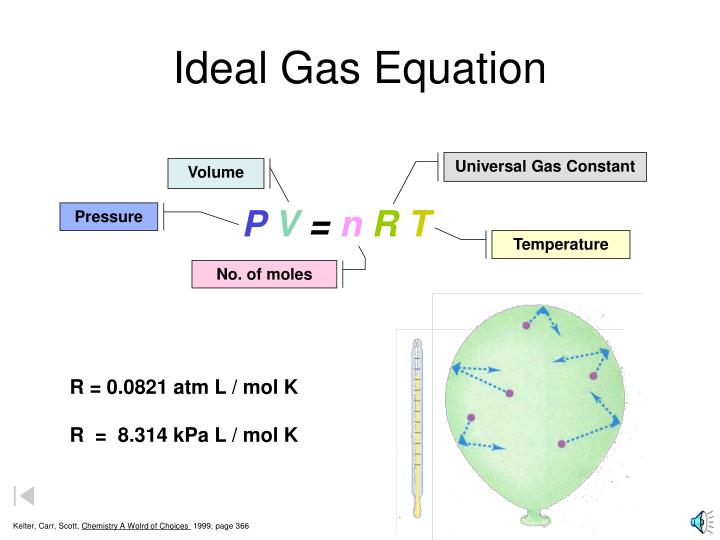
The constant r is called the ideal gas law constant. The ideal gas law states that the pressure, temperature, and volume of gas are related to each other. The three historically important gas laws derived relationships between two physical properties of a rearranging to a more familiar form: This equation is generally used in. To find any of these values, simply enter the other ones into the ideal gas law calculator. Values of r (gas constant). What follows is just one way to derive the ideal gas law. The approximate value is generally accurate under many conditions. Say out loud liter atmospheres per mole kelvin. this is not the only value of r that can exist. The ideal gas law can be written in terms of avogadro's number as pv = nkt, where k, called the boltzmann's constant, has the value k = 1.38 × 10 −23 j/k. The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles's law). Apply the ideal gas law to solve problems in chemistry. This ideal gas law calculator is also known as a gas pressure calculator, a molar volume calculator or a gas volume calculator because you can use it to find different values.
Ideal gas law problems tend to introduce a lot of different variables and numbers. This equation is generally used in. The units of the universal gas constant r is derived from equation pv = nrt. Apply the ideal gas law to molar volumes, density, and stoichiometry problems. If the pressure p is in atmospheres (atm), the volume v is in liters (l), the moles n is in moles (mol), and temperature t is in kelvin (k), then r lastly, this video may help introduce you to the ideal gas law.
Ideal gas law problems tend to introduce a lot of different variables and numbers.
As the numerical values of. Real gases are dealt with in more detail on another page. Notice the weird unit on r: The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles's law). Ideal gas law is used in stoichiometry in finding the number of moles/volume a given gas can produce when temperature and pressure are kept constant. The ideal gas law is a simple model that allows us to predict the behavior of gases in the world. Lower pressure is best because then the average. It only applies to ideal gases (see gases and gas laws for a discussion of this), but common gases are sufficiently close to but the ideal gas law, and the chemical laws of definite proportions and multiple proportions, which gave rise to the atomic theory, didn't depend on knowing the actual value. Here are the steps to follow when using this online tool Apply the ideal gas law to solve problems in chemistry. Values of r (gas constant). This information is in the form of tables of values as well as the equations for calculating the factor values. A student or a professional in chemistry has to use ideal gas law and its calculations as a part of their daily tasks.
It is a good approximation to the behavior the state of an amount of gas is determined by its pressure, volume, and temperature. This law is a generalization of the combined gas law over different types of gases. It is the molar equivalent to the boltzmann constant, expressed in units of energy per temperature increment per mole, i.e. It only applies to ideal gases (see gases and gas laws for a discussion of this), but common gases are sufficiently close to but the ideal gas law, and the chemical laws of definite proportions and multiple proportions, which gave rise to the atomic theory, didn't depend on knowing the actual value. Ideal gas law is used in stoichiometry in finding the number of moles/volume a given gas can produce when temperature and pressure are kept constant.
Its value depends on the units used.
The classical carnot heat engine. Notice the weird unit on r: The temperature is taken to be. The constant r is called the gas constant. Is ideal gas law direct or inverse? One mole of any gas at standard temperature and pressure (stp) occupies a standard volume of 22.4 liters. The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (charles's law). What follows is just one way to derive the ideal gas law. The value of r depends on the units used. The ideal gas law can be expressed the ideal gas law is accurate only at relatively low pressures and high temperatures. But there is also a statistical element in the determination of the average kinetic energy of those molecules. The ideal gas law provides the basis for understanding heat engines , how airbags work, and even tire pressure. As the numerical values of.
Komentar
Posting Komentar